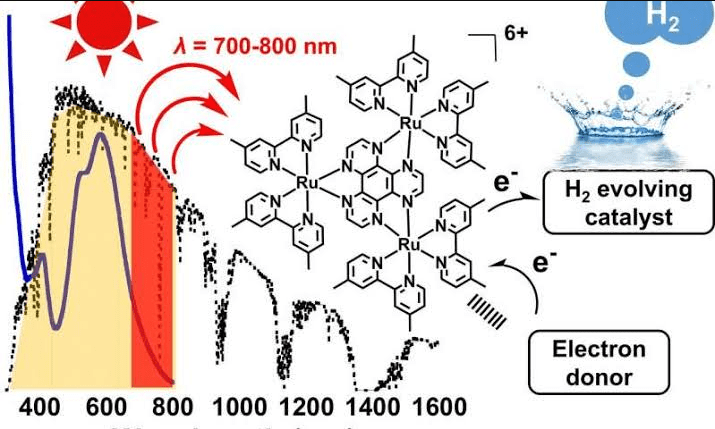
Claudia Turro and her colleagues Whittemore, Xue, Huang, and Gallucci from Ohio State University presented a rhodium catalyst that achieves a previously unattained high efficiency in the generation of hydrogen from water and light.
In contrast to catalysts already found, it can use the entire light spectrum of the sun, including the least energetic red and infrared components. In the field of basic research, the increased efficiency is already a major step forward – even if it is still a long way from an application. Firstly, they want to move away from expensive rhodium, and secondly, the model has so far only worked with acid as a proton supplier, but not with pure water.
Thinking with H2
The concept could fit the energy turnaround in a broader sense. If electricity is increasingly to come from renewable sources in the future, appropriate possibilities for storing and distributing energy are needed to ensure the security of supply. Hydrogen as a possible energy carrier has a fixed role in the thought processes. It could store excess electricity on windy and/or sunny days and, if required, generate electricity and heat in fuel cells, for example.
It could also be fed directly into the natural gas grid or mechanized, i.e. converted into synthetic natural gas. Energy suppliers could thus also supply industry with sustainable hydrogen – today it is still produced from fossil methane with the emission of CO2. Increasing sector coupling has increased efficiency and energy supply security. None of this exists today on a sufficient scale for a hydrogen economy.
Hydrogen from light can complement the H2 economy
Within certain niches of a possible hydrogen economy, it could become interesting to produce hydrogen directly from light without an efficiency-reducing detour via electricity production. Whether, from when, or for how long this is worthwhile will then determine the development of energy production. However, numerous institutes and scientists are already working on solutions today. Last year, for example, scientists at the Technical University of Ilmenau achieved an efficiency of over 19 percent in a solar cell that electrolyzes water with the electricity generated, from a maximum possible 23 percent in its concept.
Most of these approaches to solar H2 production are based on a combination of molecules for splitting into oxygen and hydrogen. The hydrogen side of this process generates excited electrons with a light-absorbing photosensitizer (in plants this is chlorophyll). These electrons are combined with two protons in a hydrogen-producing catalyst to form hydrogen gas. However, energy is lost during the charge transfer from the light absorber to the catalyst.
Two steps at once
Turro, therefore, tried to achieve both steps in the same molecule, which did not work well with the hydrogen production catalysts studied so far. They proved to be unstable, inefficient and completely inactive in the spectral range of red or infrared light. The newly developed catalyst contains two rhodium atoms bonded together, flanked by two ligand pairs, benzo [c] cinnoline and N, N-diphenylformamidate.
These contribute to the shortening of the rhodium atomic bond, thereby changing the energy level of the complex. At the same time, it extends the duration of the excited state, a key factor for its improved performance, as summarized by C&EN. According to the scientists’ publication, a single catalyst molecule should be able to produce up to 28 H2 molecules per hour under red light and an average of 170 hydrogen molecules (+/- 5) in a whole day.
Cheaper transition element sought
Basic research so far only shows that such catalysts could offer significantly higher efficiency than those in which an additional sensitizer is required. Should they ever work in the future, they will first have to be brought up to par with other methods of producing renewable hydrogen, such as using electricity from photovoltaics. The extent to which they may one day be needed will depend on the development of the whole system of energy production. However, it seems that niche applications are being reckoned with fairly firmly.
A special feature of basic research is its relative independence. Although projects on photocatalysis are financially supported, the team is taking the next tiny step without a secure perspective for a possible hydrogen economy. It is trying to replace rhodium with a less rare, cheaper transition element.